8.3: Evaluation of new platelet components for transfusion
8.3.1: Introduction
In establishing any novel component, the development process is expected to involve three stages (see Table 8.1d):
- Investigation (Phase 0): Initial intensive investigation of a range of parameters on a relatively small number of units (e.g. 10 -16) to establish concepts. This should involve in vitro studies with serial sampling, and may also involve in vivo studies. Components produced during this phase should not be used for transfusion. For clarity the guidance on which tests need to be performed is as shown in Tables 8.3a and 8.3b.
- Validation (Phase 1/Phase 2): Operational validation on a larger number of units (e.g. 125) to establish routine operation of the technique, normally testing for those parameters listed in the current edition of the Red Book. These tests may be supplemented by a limited set of assays selected from the investigational phase to allow setting of routine quality parameters. This may involve in vivo studies and normally would involve sampling at the times shown below for routine testing.
- Routine (Local process validation): Ongoing routine validation using a small set of parameters selected on the basis of the above studies. This will not normally involve in vivo studies. Advice may be sought from SACBC on the validation requirements for platelets produced from automated processing of whole blood or buffy coats or other technologies that are not specified in Tables 8.3a and 8.3b.
Platelet components may be derived from whole blood using platelet-rich plasma or buffy coat methods of preparation, or by plateletpheresis and, in either case, the standard requirements for donor selection and for mandatory donation microbiological testing should be fulfilled. For components prepared in a closed system, storage in specifically designed plastic bags is currently undertaken with gentle agitation for up to 7 days at 22 ±2°C. The platelet concentration should not exceed the platelet bag manufacturer’s recommendation. Platelet components may be subjected to leucodepletion, storage in platelet additive solutions in place of plasma and, in the case of whole blood derived components, pooling of four to six units to form an adult equivalent dose. When well prepared, there is no evidence that the clinical performance of any of these products is different, and the guidance provided below applies equally to the various approaches.
In vitro assays should be performed on samples representative of the pack contents taken by an aseptic technique that does not appreciably alter the gross volume of the pack contents (must be kept to a minimum but in any case no greater than 10%) on Days 1, 3, 5 and 7 (and further samples if an extension of shelf life is proposed or for components that have a shorter shelf life). For studies investigating an extension to shelf life, consideration should be given to testing the component 1 day after the proposed limit of shelf life (e.g. Day 8 for a 7-day shelf life). Parallel testing of units prepared by a well-established method is recommended, and the use of a split-pool or crossover design will increase the power of such comparisons. In vivo studies, preferably with parallel testing of ‘standard’ components, should be performed on the last day of the proposed storage period. The number of units to be studied should be based on the study objectives and design and determined by statistical analysis based on the difference between test and control units to be detected. A sample size of ten tests or controls would be required to detect a 30% difference in pH and CD62P at Day 7 of storage using an unpaired study. Fewer units will be required if a pooled and split study design is used.
8.3.2: Investigational phase
8.3.2.1: Guidance
Table 8.3a recommends an assessment format for different kinds of novel development that may be expected for platelet components. While these are listed against the recommended assays above, this is not intended to be restrictive and comparable alternatives may be employed. It is recommended that any protocol for the evaluation of a novel blood component or production method be discussed with the Chair of the SACBC before finalisation.
For leucodepleted components, leucocyte enumeration should involve validated techniques and would currently be expected to exhibit a sensitivity of less than or equal to 1 leucocyte per microlitre.
8.3.3: In vitro assessment
8.3.3.1: Background
In vitro assessments essentially use surrogate assays that are hoped to be indicative of the in vivo performance of platelets, as measured by haemostatic effect, in vivo recovery and survival and corrected count increment following transfusion. While a large number of in vitro assays have been proposed, only a few of these have been shown to correlate with post-transfusion indices. This area has been reviewed by the BEST group1 and can be summarised in Table 8.3a (* = correlates with in vivo viability).
Any platelet production system that may be considered as having the potential for an increased risk of bacterial contamination or growth should include an assessment of sterility as part of the initial validation phase. It is recommended that at least 50 apheresis units or pools (each sufficient for a standard adult dose) should be assessed for sterility by a validated technique prior to in vivo assessment and routine introduction of the component into clinical use.
Consideration should be given when performing in vitro studies to including periods where platelets are temporarily not agitated to reflect current practice such as during transportation.
Novel Plasticisers (please also refer to Tables 8.3a, 8.3b and section 8.8 for further guidance):
Where novel plasticisers are used, the levels of recovered plasticiser should be monitored over shelf life to assess the levels of leaching into the blood component. Blood bag manufacturers or external laboratories may be required for chemical analysis of plasticisers. Methodology will be specific to the plasticiser under investigation, but likely to be by liquid chromatography-mass spectrometry. Advice can be sought from manufacturers, SACBC and peer-reviewed literature. It is also important to consider metabolites that may also influence product quality and may have toxicological effects. Concentrations in the supernatant and platelets should be measured at the beginning, during and end of storage to assess leaching and potential patient exposure. Consideration must be given to the effects of irradiation on the bag and subsequent leaching potential. Suppliers must undertake toxicology studies as part of CE/UKCA/UKNI marking. Suppliers must provide evidence of an independent review of toxicology data; this data will then be reviewed by SACBC.
Novel Additive solutions (please also refer to section 8.8 for further guidance):
Where novel additive solutions are used, effects on storage must also be taken into account. Table 8.3b provides a list of recommended tests. Where there are combinations of novel elements to a blood bag system (e.g. novel plasticiser and additive solution), then Table 8.3a and 8.3b should be used to ensure requirements for each element is included within the minimum recommended tests. There will likely be overlap in requirements and SACBC can provide advice on this if required.
Table 8.3a In vitro assessment
| Recommended | Alternatives (may be used if validated against parameters that correlate with in vivo viability) | |
|---|---|---|
|
(a) |
Product content Volume Platelet content Leucocyte content Plasma content (for additive developments only) |
|
|
(b) |
Platelet morphology (proportion of discs) Determination of swirling Morphology index (phase microscopy)* Extent of shape change by ADP* |
|
|
(c) |
Platelet metabolism ATP* Hypotonic shock response* pO2/pCO2pH Glucose consumption Lactate production |
|
|
(d) |
Extent of platelet activation |
Surface GPIb/IX (CD42a/42b) |
|
(e) |
Extent of platelet lysis Supernatant lactate dehydrogenase |
Soluble annexin V |
|
(f) |
Measurements reflecting in vitro function Aggregation in response to paired antagonists (e.g. 80 µM ADP and 8 µg/mL collagen) |
In vitro bleeding time (in development) Platelet adhesion (e.g. Baumgartner) |
|
(g) |
Assays indicative of possible side effects |
|
8.3.4: In vivo assessment
If in vivo assessment is required local ethical committee approval should be obtained prior to commencing the in vivo assessment.
Additional measurements at 4–6 and/or 24 hours post-transfusion may give some indication of platelet survival.
Any in vivo assessments should be performed at the end of the proposed storage period, following generation of sufficiently reassuring data from in vitro studies. For studies investigating an extension to shelf life, consideration should be given to testing the component 1 day after the proposed limit of shelf life (e.g. Day 8 for a 7-day shelf life). Due to the inherent variability of patients, use of a crossover design or dual labelling technique in stable, afebrile thrombocytopenic patients without evidence of platelet consumption (or in volunteers) is strongly recommended so that each patient acts as their own control. The number of components transfused should be justified on the basis of the study objectives and design.
Platelet counts should be assessed immediately prior to infusion of an appropriate dose of ABO identical platelets and 1 hour post-infusion.
Two approaches are established:
- Use of radioisotope-labelled platelets in normal volunteers for determination of platelet recovery and survival: This approach is not applicable to pooled products. Validated techniques must be used.
- Determination of recovery after transfusion: An appropriate adult dose (>240 × 109 platelets) of ABO identical platelets may be used to determine increments and therapeutic effect (bleeding time measurements are not recommended). Patients known or suspected to have lymphocytotoxic or human platelet antigen (HPA) antibodies should be excluded and should have no evidence of hypersplenism, sepsis, ongoing haemorrhage or other cause of increased platelet consumption.
Table 8.3b Evaluation of new platelet components for transfusion
| Parameter | Leuco-depletion | Pathogen reduction | Extended storage | Sterile connection | New bag, additive or anticoagulant | Novel plasticiser/plastic |
|---|---|---|---|---|---|---|
|
Volume (d1) |
|
|
|
|
|
|
|
Platelet content |
|
|
|
|
|
|
| Red cell count | 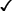 |
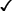 |
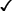 |
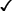 |
|
|
|
Leucocyte content (d1) |
|
? |
? |
|
? |
|
|
Leucocyte subsets (%) |
? |
? |
? |
|
? |
|
| Plasma : PAS ratio | 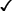 |
|
||||
| Plasma content | 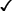 |
|
||||
|
Morphology, e.g. Swirl test |
|
|
|
|
|
|
|
Activation, e.g. beta thromboglobulin, CD62P |
|
|
|
|
|
|
|
Lysis, e.g. lactate dehydrogenase |
|
|
|
|
|
|
|
Metabolic activity, e.g. ATP, pH, Lactate, Glucose, |
|
|
|
|
|
|
|
Function e.g. Aggregation |
? |
? |
? |
? |
? |
|
|
Cytokines/chemokines |
|
|
|
|
|
|
|
FXIIa |
? |
? |
|
|
? |
|
|
Sterility |
if dock on |
|
|
|
? |
|
|
PrPc and microvesicles |
? |
|
|
|
|
|
|
Pathogen reduction* |
? |
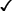 |
|
|
|
|
| Recovered plasticiser in supernatant and plasma | 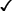 † † |
|||||
|
Key: |
||||||