26.2: Specification
The layout of the blood pack base label is shown in Figure 26.1 and is divided into three areas. Area numbers on the diagram are for reference purposes only and must not be reproduced on the label. Where volumes are to be specified on the base label they must be given in millilitres. Anticoagulant and additive formulations must be in English.
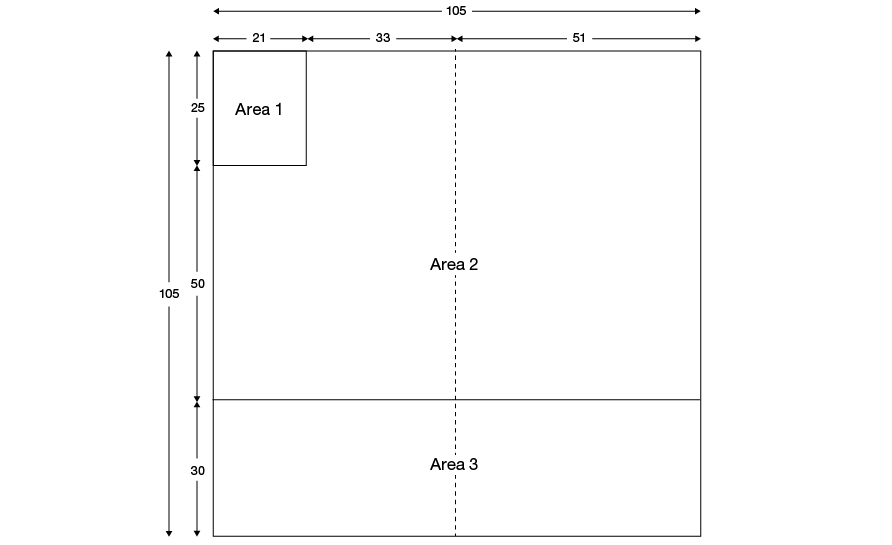
Figure 26.1 Base label layout: dimensions in millimetres for 400 mL to 600 mL pack
The manufacturer’s logo may be printed in a colour of their choice. All other printing must be black on white. Labels, adhesive and ink must comply with the requirements of the current version of the International Organization for Standardization Standard ISO 3826-1, Plastics collapsible containers for human blood and blood components – Part 1: Conventional containers.
Area 1 is provided for the manufacturer to print their logo, name and address.
Area 2 is provided for the manufacturer for the following information concerning the medical device. (Note: this area is typically covered after application of Blood Transfusion Service overstick labelling.)
- On all packs the words or approved medical device symbol(s) shown in Figure 26.2:
- Do not re-use this container
- Do not vent
- Contains phthalate e.g. DEHP (if applicable)
- Sterile
- Pyrogen free fluid pathway
- Do not use if damaged/deteriorated
- Expiry date (see note below)
- Refer to instructions for use
- On all packs the ‘CE’ mark and the registration number.
- On primary blood collection packs, the formulation and volume of anticoagulant.
- On additive packs, the formulation and volume of the additive solution.
- The nominal volume of blood/component that is to be collected into the container. (Instructions for use should also give the volume range.)
- Where a pack is specifically intended for the storage of a particular component, the identity of that component, e.g. ‘Suitable for the storage of platelets’, must be labelled. Alternatively this information can be provided by symbols in the current version of ISO 3826-2 Plastics collapsible containers for human blood and blood components – Part 2: Graphic symbols for use on labels and instruction leaflets.
- Manufacturers should, where appropriate, indicate that the blood pack including its sub-components are latex free. The symbol in Figure 26.2 may be used.
Note: The expiry date may be expressed as: ‘Do not use after DD/MM/YY’ where DD is the day number, MM the month number and YY the year. For clarity, the expiry date will be midnight (23:59 hours) on the date shown. It is permissible to use only MM/YY; in this instance the expiry date will be midnight on the last day of the month/year shown.
The expiry date may alternatively be provided by the manufacturer on the immediate overwrap in which case the following statement must be applied to the base label: ‘Use within x days of opening the immediate overwrap’, where x is the number of days validated by the manufacturer.
Labelling of the blood pack overwrap and packaging must comply with the current versions of ISO 3826-1 and the EU Medical Devices Directive.
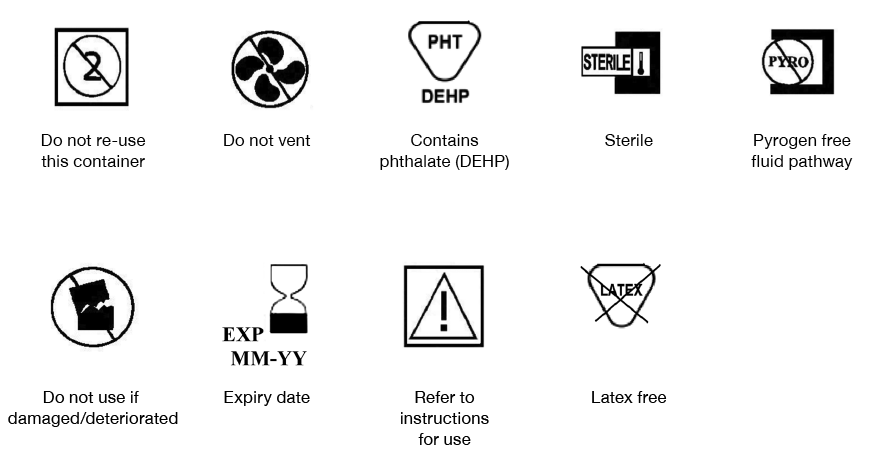
Figure 26.2 Symbols used on blood packs and on critical consumables (ISO 3826-2, ISO 15223-1 and EN 980)
Area 3 is provided for the manufacturer’s pack type (list/catalogue) and pack batch or lot number. This information must be printed in both eye-readable and barcoded formats. The UK Blood Transfusion Services are currently in transition from ABC Codabar to ISBT 128 for the provision of this information. Details of both conventions are provided below. The manufacturer’s pack type and lot number must remain visible in eye-readable format after application of overstick labels.
Labels must, after sterilisation, conform to the dimensions specified in Table 26.1.
Labelling of the blood pack overwrap and packaging must comply with the current versions of ISO 3826-1 and EU Medical Devices Directive.
Label adhesives applied directly to the plastic film of a blood bag must be tested and approved by manufacturers in accordance with the relevant current versions from the ISO 10993 biocompatibility series of standards (or equivalent national standards). The risk of the adhesive coming into contact with blood or blood components should be established by ‘extractables’ testing (ISO 10993 – Parts 1, 17 and 18). If testing reveals an unacceptable level of migration, biocompatibility testing should be extended to interaction with blood (ISO 10993 – Part 4) and toxicological testing (ISO 10993 – Parts 3 and 5).
- ISO 10993-1:2009 Biological evaluation of medical devices. Part 1: Evaluation and testing within a risk management process
- ISO 10993-3:2003 Biological evaluation of medical devices. Part 3: Tests for genotoxicity, carcinogenicity and reproductive toxicity
- ISO 10993-4:2002/Amd 1:2006 Biological evaluation of medical devices. Part 4: Selection of tests for interactions with blood
- ISO 10993-5:2009 Biological evaluation of medical devices. Part 5: Tests for in vitro cytotoxicity
- ISO 10993-17:2002 Biological evaluation of medical devices. Part 17: Establishment of allowable limits for leachable substances
- ISO 10993-18:2005 Biological evaluation of medical devices. Part 18: Chemical characterisation of materials.
Table 26.1 Blood bag base label dimensions (width × depth)
| 100 mL bag |
60 × 85 ±5 mm* |
| 250 mL bag |
90 × 85 ±5 mm* |
| 400 mL bag |
105 × 105 ±5 mm |
| 500 mL bag |
105 × 105 ±5 mm |
| 600 mL bag |
105 × 105 ±5 mm |
| * The layout of base labels applied to smaller packs must be controlled within the purchasing specification. Barcode positions are indicated in the ISBT 128 (ICCBBA) product labelling section. |
|