24.4: Tissue product labels
24.4.1: The base label
The base label dimensions are to be at least 110 mm wide by 104 mm high on all tissue containers/labels where the square format is used. Where the horizontal format is used they will be at least 220 mm wide by 52 mm high.
It is noted that where tissues are stored in very small containers, the base label may be attached to a secondary container. In these circumstances, the inner container will be labelled with the donation number barcode to provide a link to the label on the outer packaging.
The guide marks on Figures 24.3 and 24.4 are required to assist positioning of later labels.
24.4.1.1: Square format
The top left-hand quadrant will be used for the application of a donation identification number label. The space below this on the left-hand side of the label between 25 mm and 40 mm from the top edge must be left blank. This space may be pre-printed, for certain donations, by the company supplying the container with the lot/batch number of the container. This text must be visible at all times.
The bottom left-hand quadrant will be overstuck with a product description label.
The top right-hand quadrant will have pre-printed text indicating that the donation is in quarantine, as follows:
QUARANTINE
NOT YET RELEASED FOR CLINICAL USE
The top right-hand quadrant will be overstuck with a status label once its status has been determined (for cryopreserved products, this quadrant can be overstuck with a ‘See outer container for product status’ label). The status label can then be applied to a secondary container.
The bottom right-hand quadrant will be overstuck with an expiry date label which relates to expiry date prior to issue or to expiry date following issue.
24.4.1.2: Horizontal format
The same four quadrants will be used but the order in this case will be as shown in Figure 24.4.
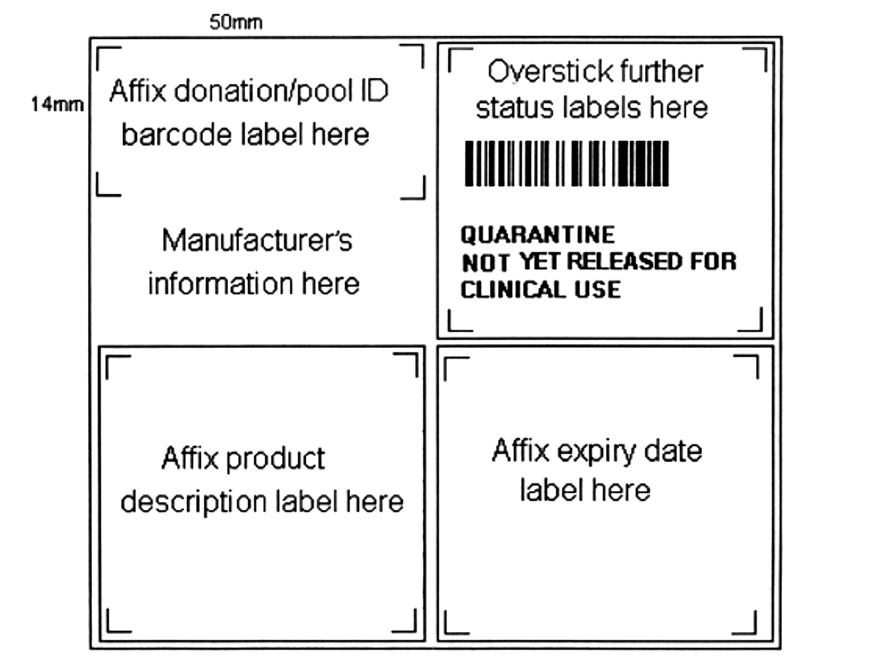
Figure 24.3 Base label design: square format
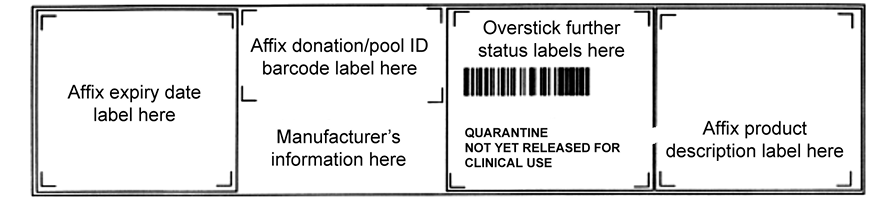
Figure 24.4 Base label design: horizontal format (not to scale)
24.4.2: Donation and pool identification number labels
The donation identification number plays a critical role in the safety of the tissue supply. It provides a unique identification number which cross-references tissue donations and products and samples taken at the time of donation. Where tissue products are not further processed, they are issued with the same donation identification number label.
Donation identification number labels must be generated in sets under strictly controlled conditions which ensure that all the labels in a set bear the same number, and that each set is unique. It is the responsibility of the manufacturer of the label sets to undertake appropriate quality control measures to ensure these conditions are met.

Figure 24.5 Donation identification number label set
Pool identification number labels are generated on demand when donations are processed together, whether from one or more donors.
As tissues are usually transferred from their original containers to secondary and tertiary containers during processing, it is a requirement that donation and pool identification numbers can be printed on demand.
An example set of identification numbers is shown in Figure 24.5. Barcode density information is provided in the ISBT 128 Technical Specification. The structure of the donation identification number is described further below.
For identification number labels to be applied to tissue products, the dimensions of the label are as indicated in Figure 24.6. As with all barcodes, there should be a minimum ‘quiet zone’ of 2.5 mm between the edge of the label and the start of the barcode. This label is affixed to the top left-hand section of the base label.
The donation identification number complies with ICCBBA Data Structure 001. The country/collection facility identification codes included within this data structure are allocated by ICCBBA and a list of these can be downloaded from ICCBBA’s website. Any tissue service site within the UK requiring a new facility code must make a request first to SACIT before applying to ICCBBA for registration.
The collection year characters (6 and 7) should correspond to the last two digits of the year in which collection took place. In practice, this cannot be readily achieved using pre-printed labels without considerable wastage. Within the UK it is therefore permissible to allow a maximum variation of 1 month either way, i.e. it is permissible to use the previous year’s collection year characters up to 31 January in the current year, and to use donation numbers with the following year’s collection year characters from 1 December in the current year.
The use of ICCBBA Data Structure 001 allows either an inclusion of the modulus 37,2 check digits or the ability to use the last two characters as process control flags. At present all UK donation identification numbers incorporate the modulus 37,2 check digits. If the check digits are not used and process control flags are used in their place, they must be registered and authorised by SACIT prior to their use.
The algorithm for calculating 37,2 check characters can be found in the ICCBBA ISBT 128 Technical Specification. Users will need to take into account that units imported from countries outside the UK may well use these flag characters for process control as permitted in the specification.

Figure 24.6 Donation number label dimensions

Figure 24.7 Donation number showing process control/flags characters

Figure 24.8 Form boxes designed to enable accurate recording
The eye-readable format of the donation identification number in the UK comprises the data characters excluding the flag characters, followed by the manual entry check character. The layout differs from that in the ICCBBA ISBT 128 Technical Specification in that all characters of the number must be of equal size and weight. Printing of the six-digit unit serial number in larger or bold characters is not permitted. Software manufacturers should ensure that only the eye-readable format is presented in screen displays.
If process control flags are used within the donation identification number, they must be displayed as per the standard between the last digit and the boxed eye-readable check digit (i.e. 90 degrees to the rest of the number) as shown in Figure 24.7.
The number should be displayed with the characters grouped in a 4,3,3,3,1 arrangement. It is recommended that the check character be enclosed in a box where this is possible. The check character set uses the characters I and 1, 0 and O, and the font selected should be one which allows easy differentiation between these characters.
Where the donation identification number has to be recorded manually, form designs that assist accurate recording, such as the use of boxes to encourage correct character grouping, are recommended. An example is given in Figure 24.8.
The full eye-readable donation identification number, including check character, must be recorded and entered in all cases. The use of pre-programmed shortcut keys (‘hot’ keys) or pre-printing of part of a number is not acceptable.
24.4.3: The product label
The product label (50–55 mm wide by 50–52 mm high) is affixed to the left lower quadrant of the square base label or the right-hand side of the horizontal base label, if printed as a single quadrant (it may be printed as a combined label with another quadrant). A start product label is attached to all base labels at the time of tissue retrieval. If the tissue remains in the same container until the time of issue for implantation, it is issued with this product label. If it is subjected to further processing while remaining in its original container (e.g. gamma irradiation) the start product label is overstuck with the appropriate final product label before application of a status label. If it is transferred to another container following processing, a new base label is attached and the appropriate final product label is attached to the lower half of the base label following processing. The template for the product label is shown in Figure 24.9.
Codes for tissues and tissue products should only be used if they are registered with ICCBBA, approved by the Standing Advisory Committee on Tissues and Cellular Therapy Products (SACTCTP) and the Standing Advisory Committee on Information Technology (SACIT).
New tissue donations and tissue products will have codes assigned as required by SACIT in liaison with SACTCTP and ICCBBA. Requests for new codes should be made in writing or by e-mail to the Chair of SACIT with notification to the Chair of SACTCTP.
Updates of the Human Tissue Code Database can be obtained from the ICCBBA website (www.iccbba.org).
The first two lines of text contain the tissue component class and modifier, for example:
CORTICO-CANCELLOUS BONE RING
Freeze-dried
Lines 3 to 7 describe various attributes, where relevant, though one of these can be used to provide further product description information (see section 24.4.5). It is not necessary to include the unit of issue attribute details. Space will be available next to the method of sterilisation attribute where exposure ‘dots’ can be applied. The significance of the dot colour need not be detailed on the label but can be explained in the package insert. The volume/dimension field will contain either the actual or nominal tissue volume or other relevant dimensions (e.g. length, depth etc.). Immediately under the product barcode will be the unique reference number of the label which will correspond to the eye-readable barcode without the data identifier characters.
An example of a product label is given in Figure 24.10.
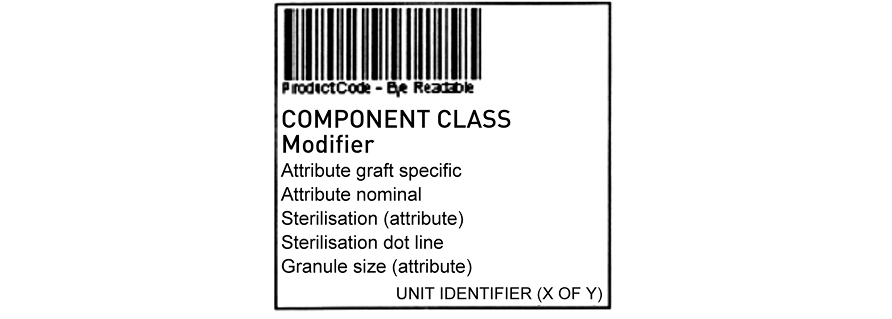
Figure 24.9 Tissue product label template

Figure 24.10 Product label (example)
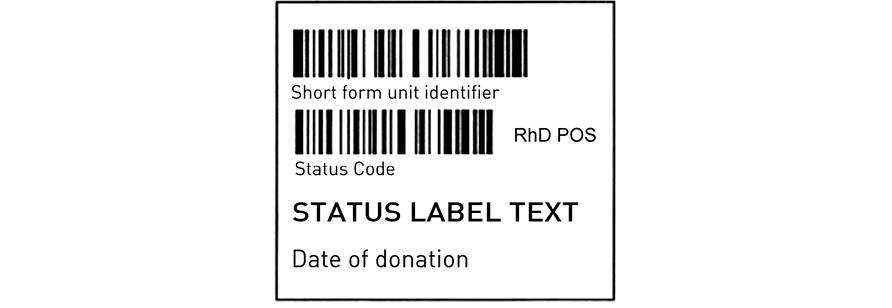
Figure 24.11 Tissue release status label

Figure 24.12 Status label (example)
24.4.4: The tissue status label
The tissue status label is a demand-printed label (50–55 mm wide by 50–52 mm high). The overall layout of the label is shown in Figure 24.11. This label can include graft-specific information such as date and site of donation.
An example of a status label is given in Figure 24.12.
The elements of the label content from top to bottom are described in the following sections.
24.4.4.1: Non-ICCBBA defined data structure – unit identifier (short form ID)
The tissue status label is unique to a specific donation. To ensure that it is affixed to the correct container a barcode, the nationally defined identification code, is printed on the label. This is used by the Service in a concatenated read with the donation identification number at the time of labelling. (Note: Although this is a non-ICCBBA defined data structure, it is ICCBBA authorised and controlled in the UK through the SACIT committee.)
The structure of the short form unit identifier is defined in section 24.3.5. Either the ‘&a’ or ‘&b’ versions of the code can be used.
Barcode indicating product status and the statement of product status
The statement of product status (which appears below the barcode) will be one of those listed in Table 24.1.
The barcode giving the product status is taken from the ICCBBA ISBT 128 Technical Specification.
24.4.4.2: Donation-specific information
Specific information which applies to the donation may be included on this label. This includes the RhD blood group and the date of donation.
The RhD blood group is only relevant where red cells remain in the final tissue product and where the product is supplied for female recipients of childbearing age.
Below the short form unit identifier is printed the RhD blood group barcode for certain products. The text relating to RhD type will be printed to the right of the status barcode. Where the RhD type is indicated, products will always be fit for clinical use.
Within the UK the blood group data structure will reflect that given in the ICCBAA Data Structure 002:
=%ggre
Where:
‘gg’ is the ABO/Rh/status code. The default ABO/Rh codes or the special message codes described in the ICCBBA ISBT 128 Technical Specification will be used as indicated. The characters r and e from this data structure are not used in the UK at this time and are both set to 0 (i.e. 00).
For example, an RhD positive unit will code as:
=%T100
The barcode and text content of these labels is described in section 24.4.5.
Date of donation can also be included on this label following the conventions described for expiry dates. This information does not need to be barcoded; it is represented in text only and must comprise the day number, the month represented by its first three characters, and the four-digit year (e.g. 1 FEB 2002).
24.4.5: Status label definitions
For each of the special message codes indicated in the status label section an associated status label is defined. The specification for these labels is divided into two sections: one for essential information which must be present on the label as specified, and one for optional information which may be added if desired. All labels are to be demand-printed black on white.
Table 24.1 Statements of product status
| Statement | Status code |
|---|---|
|
FIT FOR CLINICAL USE (RhD NOT DEFINED) |
T3 |
|
QUARANTINE NOT YET RELEASED FOR CLINICAL USE |
Mq |
|
RhD POS FIT FOR CLINICAL USE |
T1 |
|
RhD NEG FIT FOR CLINICAL USE |
T2 |
|
MUST BE STERILIZED |
T6 |
|
BIOHAZARDOUS |
Mb |
|
DISCARD |
Md |
|
FOR IN VITRO R & D ONLY |
Mr |
|
SEE OUTER CONTAINER FOR PRODUCT STATUS |
T5 |
|
AUTOLOGOUS USE FIT FOR CLINICAL USE |
Ma |
|
AUTOLOGOUS USE QUARANTINE |
T4 |
24.4.5.1: T3 ‘FIT FOR CLINICAL USE’ (RhD not specified)
Essential information
Barcode: ISBT 128 group code, where gg = ‘T3’. Positioned to allow concatenated read with an adjacent donation number
Text: The words ‘FIT FOR CLINICAL USE’ in upper-case letters of minimum height 4 mm.
24.4.5.2: Mq ‘QUARANTINE – NOT YET AVAILABLE FOR CLINICAL USE’
Essential information
Barcode: ISBT 128 group code where gg = ‘Mq’. Positioned to allow concatenated read with an adjacent donation number
Text: The words ‘QUARANTINE’ in upper-case letters of minimum height 6 mm
Text: The words ‘NOT YET RELEASED FOR CLINICAL USE’ in upper-case letters of minimum height 3 mm.
Optional information
Text: The word ‘Reason:’ followed by a free-format message giving the reason for hold.
24.4.5.3: T1 ‘RH D POS – FIT FOR CLINICAL USE’
Essential information
Barcode: ISBT 128 group code where gg = ‘T1’. Positioned to allow concatenated read with an adjacent donation number
Text: The words ‘RhD POS’ in upper-case letters (except the ‘h’) of minimum height 6 mm to appear to the right of the status barcode
Text: The words ‘FIT FOR CLINICAL USE’ in upper-case letters of minimum height 3 mm.
24.4.5.4: T2 ‘RhD NEG – FIT FOR CLINICAL USE’
Essential information
Barcode: ISBT 128 group code where gg = ‘T2’. Positioned to allow concatenated read with an adjacent donation number
Text: The words ‘RhD NEG’ in upper-case letters (except the ‘h’) of minimum height 6 mm to appear to the right of the status barcode
Text: The words ‘FIT FOR CLINICAL USE’ in upper-case letters of minimum height 3 mm.
24.4.5.5: T6 ‘MUST BE STERILIZED’
Essential information
Barcode: ISBT 128 group code where gg = ‘T6’. Positioned to allow concatenated read with an adjacent donation number
Text: The words ‘MUST BE STERILIZED’ in upper-case letters of minimum height 3 mm.
Optional information
Text: The word ‘Reason:’ followed by a free-format message.
24.4.5.6: Mb ‘BIOHAZARDOUS’
Essential information
Barcode: ISBT 128 group code where gg = ‘Mb’. Positioned to allow concatenated read with an adjacent donation number
Text: The word ‘BIOHAZARDOUS’ in upper-case letters of minimum height 4 mm
Text: The words ‘HIGH RISK’ in upper-case letters of minimum height 6 mm
Symbol: Biohazard warning symbol of minimum height 20 mm.
24.4.5.7: Md ‘DISCARD’
Essential information
Barcode: ISBT 128 group code where gg = ‘Md’. Positioned to allow concatenated read with an adjacent donation number
Text: The words ‘DISCARD’ in upper-case letters of minimum height 4 mm.
Optional information
Text: The word ‘Reason:’ followed by a free-format message.
24.4.5.8: Mr ‘FOR IN VITRO R & D ONLY’
Essential information
Barcode: ISBT 128 group code where gg = ‘Mr’. Positioned to allow concatenated read with adjacent donation number
Text: The words ‘FOR IN VITRO R & D ONLY’ in upper-case letters of minimum height 3 mm.
24.4.5.9: T5 ‘SEE OUTER CONTAINER FOR PRODUCT STATUS’
Essential information
Barcode: ISBT 128 group code where gg = ‘T5’. Positioned to allow concatenated read with adjacent donation number
Text: The words ‘SEE OUTER CONTAINER FOR PRODUCT STATUS’ in upper-case letters of minimum height 3 mm.
24.4.5.10: Ma ‘AUTOLOGOUS USE (FIT FOR CLINICAL USE)’
Essential information
Barcode: ISBT 128 group code where gg = ‘Ma’. Positioned to allow concatenated read with adjacent donation number
Text: The words ‘AUTOLOGOUS USE’ to appear in upper-case letters of minimum height 4 mm
Text: The words ‘FIT FOR CLINICAL USE’ to appear in upper-case letters of minimum height of 4 mm.
24.4.5.11: T4 ‘AUTOLOGOUS USE (QUARANTINE)’
Essential information
Barcode: ISBT 128 group code where gg = ‘T4’. Positioned to allow concatenated read with adjacent donation number
Text: The words ‘AUTOLOGOUS USE’ to appear in upper-case letters of minimum height 4 mm
Text: The words ‘QUARANTINE’ to appear in upper-case letters of minimum height 4 mm.
24.4.6: The expiry date label
An expiry date label will be applied to base labels at the time of tissue retrieval and whenever another base label is used. A final expiry date label may be applied at the time of issue if the bank follows a policy of shortening the shelf life at the time of issue.
The use of a barcoded version of expiry date is optional. If used, it should follow either ICCBBA Data Structure 004 or 005 (it should be noted that Data Structure 004 will be stood down in the near future and only Data Structure 005 will be valid).
24.4.6.1: For ICCBBA Data Structure 004
=>cyyjjj
Where => are the data identifiers, ‘c’ designates the century (e.g. 9 for 1999; 0 for 2000); ‘yy’ designates the year and ‘jjj’ is the Julian date (i.e. the number of the day in the year, e.g. 022 is 22 JAN).
24.4.6.2: For ICCBBA Data Structure 005
&>cyyjjjhhmm
Where &> are the identifiers, cyyjjj are as Data Structure 004 and hhmm signifies the hour and minutes the product expires. It should be noted the default expiry for a product with a lifespan counted in full days will be 23:59, i.e. the product will expire on the last day at 23:59.
24.4.6.3: Other information
The expiry date must be presented in eye-readable format. Additional text will follow each expiry date and will be specific for each product. For instance:
22 JAN 2003 if stored at –40°C or lower
The eye-readable text must be printed with characters of no less than 3 mm height. The content must comprise the day number, the month represented by its first three characters, and the four-digit year (e.g. 1 FEB 2002).
The use of the date format DD MMM YYYY avoids problems which may arise due to national differences in the order of the elements of numerically expressed dates. The accepted month abbreviations are JAN, FEB, MAR, APR, MAY, JUN, JUL, AUG, SEP, OCT, NOV, DEC.
The expiry date label should also include the following text:
See package insert for further information
Unit-specific product information such as product weight may also be included on the expiry date label. For example:
84 g
Where the expiry date label is printed as a quadrant label on its own it should also have the short form donation number barcode identifier (see section 24.3.5). This is not necessary where the label is printed as part of a status label (already including this identifier). An example of an expiry date label is shown in Figure 24.13.

Figure 24.13 Expiry date label (example)